Eight steps in antibody development
1. Designing of immunogens

MBL has antigen design technologies for immunogenicity enhancement of target antigens in mice through biologically modifying purified proteins prepared for immunization. With these technologies, it is possible to dramatically increase the antibody titers of certain weakly immunogenic antigens. When immunized antigens are peptides, MBL’s proprietary peptide (sequence) design system can identify and select a sequence for immunization whereby the immunization success rate should be sufficiently high. In addition, MBL has introduced MODELAGON™ (AI-assisted epitope analysis system), developed by MTI Ltd., which is capable of designing peptides based on a different principle. With these two systems, MBL is now equipped to provide efficient design services for immunogenic peptides that can induce substantially high antibody titers. A 3D modeling function was incorporated into the systems for accurate selection of functional epitopes.
2. Preparation of immunogens
MBL has obtained ISO13485 certification. The company manufactures products of recombinant and native proteins prepared with expression systems for Escherichia coli, insect cells, animal cells, etc. These techniques are applied in the preparation of proteins for use as immunogens, depending on the purpose of use and the properties of target molecules. Extensive experience and know-how obtained and accumulated through the preparation of immunogens are fed back into the development and manufacture of product components to promote further technological improvements throughout the company.
3. Selection of immune animals

Selection of suitable immunized animals
Antibody production in live animals utilizes the animal’s immune function. It may occur that host cell proteins involved in immune system response, so-called self-antigens, interfere with the immune response due to immune tolerance and the antibody titer does not increase or increases only slightly. MBL can develop monoclonal antibodies in eight species of animals, including bird species, and animals selected as most suitable for antibody production depending on the homology of target molecules and the purpose of antibody use.
Artificial lymph nodes
In 2004, ‘Nature Biotechnologies’ reported artificial lymph nodes, then MBL had introduced this technology. This technology offers the advantage of increasing antibody titers in the blood of treated mice by 10 to 100 times higher than that in normal mice. MBL utilizes this technology for preparing mice, in which diverse and high affinity antibodies against highly specific (hence difficult to prepare) target molecules are induced.
4. Monoclonal antibody production technologies
Hybridoma method
The hybridoma method is a classic method first reported in 1975. Since its introduction as one of the company’s basic technologies in 1983, MBL has utilized the method to develop a large number of monoclonal antibodies. The company’s achievements include the development of monoclonal antibodies of human, mouse, rat, hamster, and chicken origins.
Phage display technology
In 1999, MBL established a subsidiary to specialize in phage display technology, called Institute for Antibodies Co., Ltd., and this company has since engaged in technology development using phage display. In 2016, the technology was transferred to MBL by way of a business transfer. Since then, MBL has been utilizing the phage display technology as one of three core technologies, alongside the hybridoma method and SPYMEG technology.
The phage display technology is still extensively used at MBL to generate antibodies, including not only fully human monoclonal antibodies but also antibodies from animals for which the hybridoma method is not applicable. In particular, the phage display technology plays a critical role in the development of monoclonal antibodies of rabbits, chickens, and sheep.
With regard to phage-antibody libraries as sources of antibody acquisition, MBL has six types of human antibody libraries created from different races and ages, and four types of PRIME libraries (animal pre-IMmunE) (as at 1 July 2020). In addition, MBL is promoting the creation of an in-house library of antibodies derived from animals immunized with the desired target molecules.
MBL successfully isolated many patentable fully human monoclonal antibodies using phage display technology. Two antibodies of them are licensed-out to pharmaceutical companies including CNBG, a subsidiary of SINOPHARM, one of the largest state-owned Chinese pharmaceutical companies, and are being developed for various purposes by the licensing-out partners.
- What is the phage display method?
- Generation of human monoclonal antibodies by phage display method
- Press Release: CNBG and MBL have agreed to a Comprehensive Business Alliance -Establishment of Business Operations for Clinical Diagnostic Business and Therapeutic Monoclonal Antibody Development in China- (20 June 2011)
SPYMEG technology
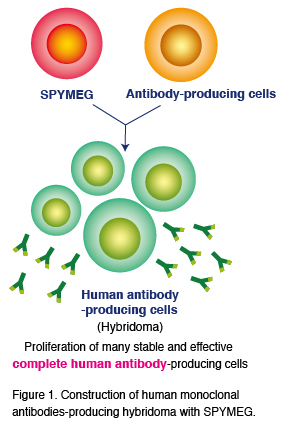
MBL succeeded in developing a novel human lymphocyte fusion partner cell line, SPYMEG. Use of SPYMEG enables acquisition of native, fully human monoclonal antibodies from human blood. Although there are several technologies reported as being feasible for the acquisition of fully human antibodies, SPYMEG is the only technology that is applicable to the hybridoma method. Since individual monoclonal antibodies are separately obtained from human cells using SPYMEG technology, the antibodies thus obtained are native antibodies that retain their functions intact in the human body. The antibodies obtained can be also used for the elucidation of mechanisms of various intractable diseases. Since the antibodies are free of artificial configurations, they are expected to be used as therapeutic drugs with minimal adverse effects if successfully developed.
The SPYMEG technology is particularly efficient in acquiring neutralizing antibodies against infectious diseases, in which antibody titers tend to increase significantly. Three antibodies isolated using this technology are licensed out to CNBG.
- Press Release: CNBG and MBL have agreed to a Comprehensive Business Alliance -Establishment of Business Operations for Clinical Diagnostic Business and Therapeutic Monoclonal Antibody Development in China- (20 June 2011)
- What is the monoclonal antibody production method?
- Human Fusion Partners SPYMEG
MAGrahd method
The magnetic-beads reaction employing the arrayed hanging-droplets method (MAGrahd method) is a basic technology for the isolation of antigen-specific antibody genes developed at the University of Toyama. In 2017, MBL acquired a use-license of the MAGrahd method from the university for use in the development and production of innovative products. A new antibody isolation system based on the MAGrahd method is the world’s fastest system for isolating and identifying target antibodies (in only five days) using gene recombination technology.
5. Screening of antibodies


MBL has technologies for efficient screening of target antibodies, comprising various hybrid systems such as affinity analysis, flow cytometry to examine cell binding, immunostaining, and cell-based assay, besides basic techniques such as ELISA, Western blot, and immunoprecipitation. MBL is also capable of performing multi-faceted analysis within the JSR group for selection of the most suitable antibodies for various measurement systems. Cell picking system: Custom-build by PMT Corp.
6. Functional analysis of antibodies
MBL can evaluate obtained antibodies for various functional analysis purposes. For example, MBL has conducted the following assessments and analyses: epitope mapping; assessment of neutralizing activity, between-species cross-reactivity, internalization activity, ADCC/CDC activity, cell invasion and migration inhibitory activities, anti-tumor and metastasis inhibitory effects, and ADC models; and analysis of the impact of glycosylation on antigen-antibody reactions, affinity, and accumulation in tumor cells.
7. Optimization of antibodies
MBL is capable of producing non-natural modified antibodies such as chimeric antibodies, humanized antibodies, scFv and Fab/F(ab)'2 antibody fragments, and bispecific antibodies by analyzing amino acid sequences of obtained antibodies. The company also has an antibody modification technology (affinity maturation by phage display technology) to dramatically improve the reactivity of antibodies.
8. Production of antibodies

MBL has rich experience in the technology used in the production of a variety of antibody subtypes, such as IgG, IgM, IgA, IgY, using hybridoma, CHO cells, and E. coli. It also manufactures in vivo grade antibodies for use in animal research.
MBL is ready to respond to requests for multi-product small lot production of antibodies for use in high-throughput screening applications and mass production of candidate antibodies for use in large-scale evaluation, and those for use in raw materials production. The capacity of large-scale production is at least 1 kg per year (theoretical) with a yield of 1-5 g/L.
Learn more about MBL’s antibody development technologies.
Strengths of MBL
- Research and development of raw materials for diagnostics
- Research and development of reagents
- Clinical performance study and pharmaceutical licensing application
- Production system
- Quality management
- Product examples








