Innovative Diagnostic Technologies
Introduction of Genetic Assay Technologies
Genetic assays encompass a wide variety of assays to obtain a broad spectrum of genetic information, including assays to predict drug efficacy and adverse reactions information from nucleic acid extracted from patient’s samples such as blood, tissue, sputum, and urine and assays to assess the presence or absence of genes of viruses and bacteria that can cause infectious diseases.
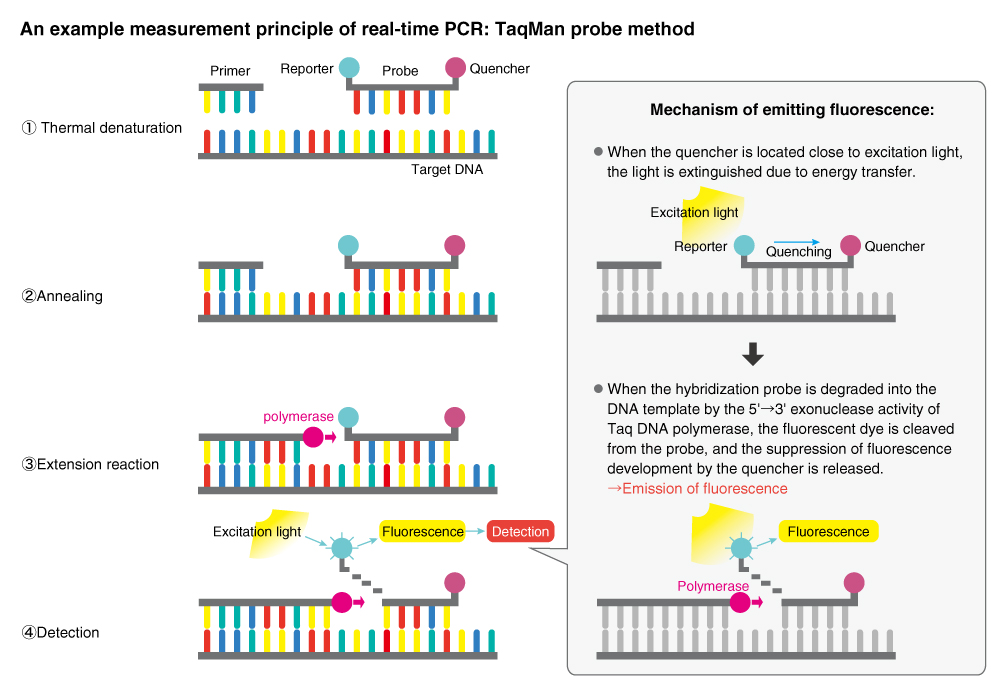
MBL is developing various clinical diagnostic reagents by applying a nucleic acid amplification method called Polymerase Chain Reaction (PCR). The technique used in real-time monitoring of amplified DNA-binding fluorescent dyes is called real-time PCR and is commonly used at assay laboratories for the diagnosis of infectious diseases, etc.
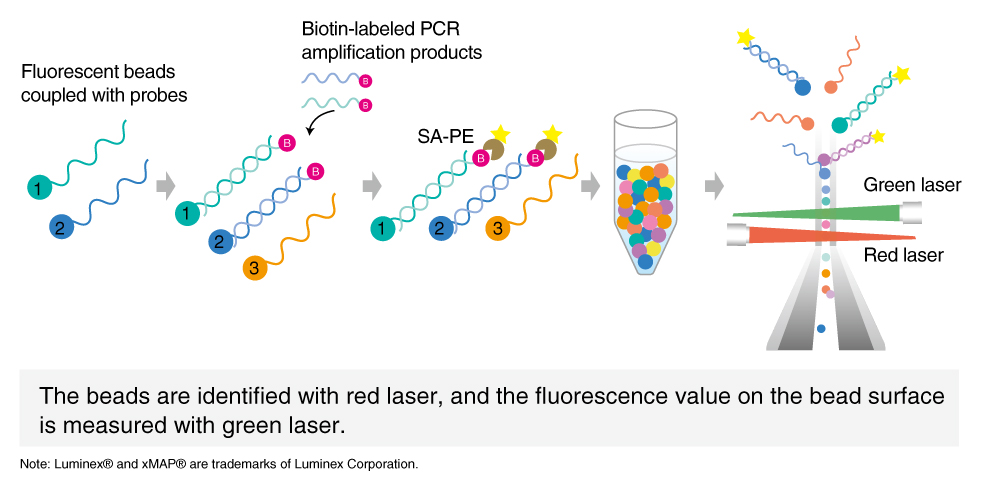
In addition, there is also a technique designed to confirm multiple gene mutations at one time to obtain a large amount of genetic information: the PCR-Luminex® method. The method is capable of simultaneously detecting genetic information of different types of mutations by using 100 types of fluorescent beads and is used for such things as genetic diagnosis of cancers and genotyping of viruses. The method is a highly useful tool for easy and simultaneous detection of dozens of gene mutations that are known to be associated with cancers.
Current genetic diagnosis sometimes requires an even greater amount of information. Advances in the Next Generation Sequence (NGS) have made it possible to obtain the complete genetic information of an organism in a short time. NGS makes it possible to perform parallel sequencing of millions of DNA fragments at once. This technology has dramatically improved gene analysis efficiency, as compared to conventional gene analysis technologies. In fact, NGS has made it possible to perform whole genome analysis of patients to support physicians’ decisions on the most suitable treatment options based on the genetic characteristics of individual patients, thereby enabling precision medicine in clinical practice.
With the purpose of providing useful information to medical professionals, MBL has started the development of novel clinical diagnostic reagents that can provide genetic information promptly and accurately for the diagnosis of cancers and infectious diseases, by employing cutting-edge technologies such as NGS.
In addition to those technologies, MBL has accumulated know-how in designing primer/probes for simultaneous detection of multiple genes in a well-balanced manner, designing reagent compositions, etc. and we are responding to the needs for reagents in clinical practice with quickness and precision.
Development of Chemiluminescent Reagents
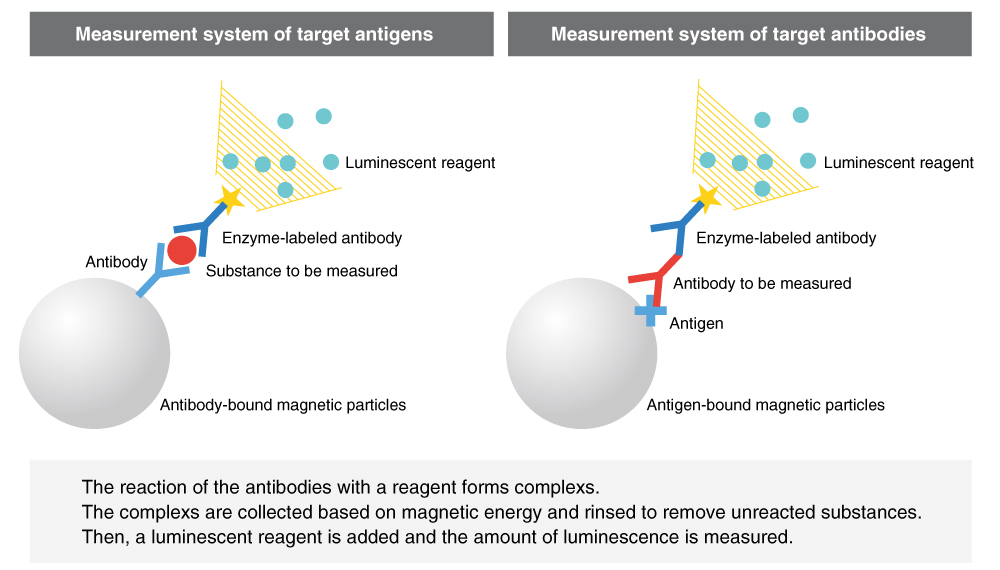
Chemiluminescent reagents are the current mainstream of immunological and serological testing reagents. These reagents are for detection of target substances in the serum through the following steps: the capture of the target substance on magnetic particles with an antigen or antibody coated onto the surface of magnetic particles, labeling of the target substance with an enzyme-labeled antibody, and luminescence with a chemiluminescent substrate. The use of magnetic particles enables automation and acceleration of assays, and detection through chemiluminescence enables the accurate measurement of target substances over a broad range, from low to high concentrations.
The development of chemiluminescent reagents starts with the development and selection of the components of the reagents, such as magnetic particles, antigens, and antibodies. MBL can select magnetic particles that have optimum surface characteristics from a list of candidate particles in close cooperation with MBL’s group company, JSR Life Science Co., Ltd. Antigens and antibodies are key raw materials that determine the accuracy and reliability of reagents. The availability of high-quality antigens and antibodies is a critical prerequisite for the market introduction of accurate and reliable reagents. MBL has strength in the development of these raw materials, and we are developing suitable raw materials for each reagent through genetic engineering approaches.
The next step is the construction of reagents with the selected raw materials. The reagents are formulated by optimizing the coating method of antigens and antibodies onto magnetic particles, determining reagent buffers necessary for appropriate particle storage and reaction, setting measurement parameters and calibration curves, etc. It is critical to formulate a reagent composition suitable for reaction with the target substance and preservation of stability during storage. Trial and error is required for the optimization of formulations.
Finally, the formulation of a reagent is tested to confirm its scientific validity, analytical performance and clinical performance. These evidence must be required for assessment of whether the reagent achieves the intended clinical benefit and safety. Particularly for in vitro diagnostic reagent, it should have clinically significant diagnostic performance.
