Looking for a CDx Partner in Japan?
Contact MBL!
MBL was founded in 1969, and since then has offered clinical diagnosis and research reagents that measure biomarkers such as disease related proteins and/or genes related to human diseases.
Over the past 50 years, we have been engaged in-house R & D, marketing, manufacturing, sales and post-sales follow-ups. We have achieved the top numbers of newly registered in vitro diagnostic products and approvals of medical reimbursement in Japan. So, we are the only one company providing "One Stop Shop" services for IVD/CDx products.
One-stop support covers development through manufacturing, sales.
You can leave flexibly whatever you want.
 |
Company Ⅰ | Company Ⅱ | Company Ⅲ | |
|---|---|---|---|---|
| Development | ||||
| Clinical performance trial | ||||
| PMDA approval | ||||
| Insurance listing | ||||
| Manufacturing | ||||
| Retailing | ||||
| Repairing |

Feel free to contact MBL!
Three reasons for why MBL
1. One Stop Shop
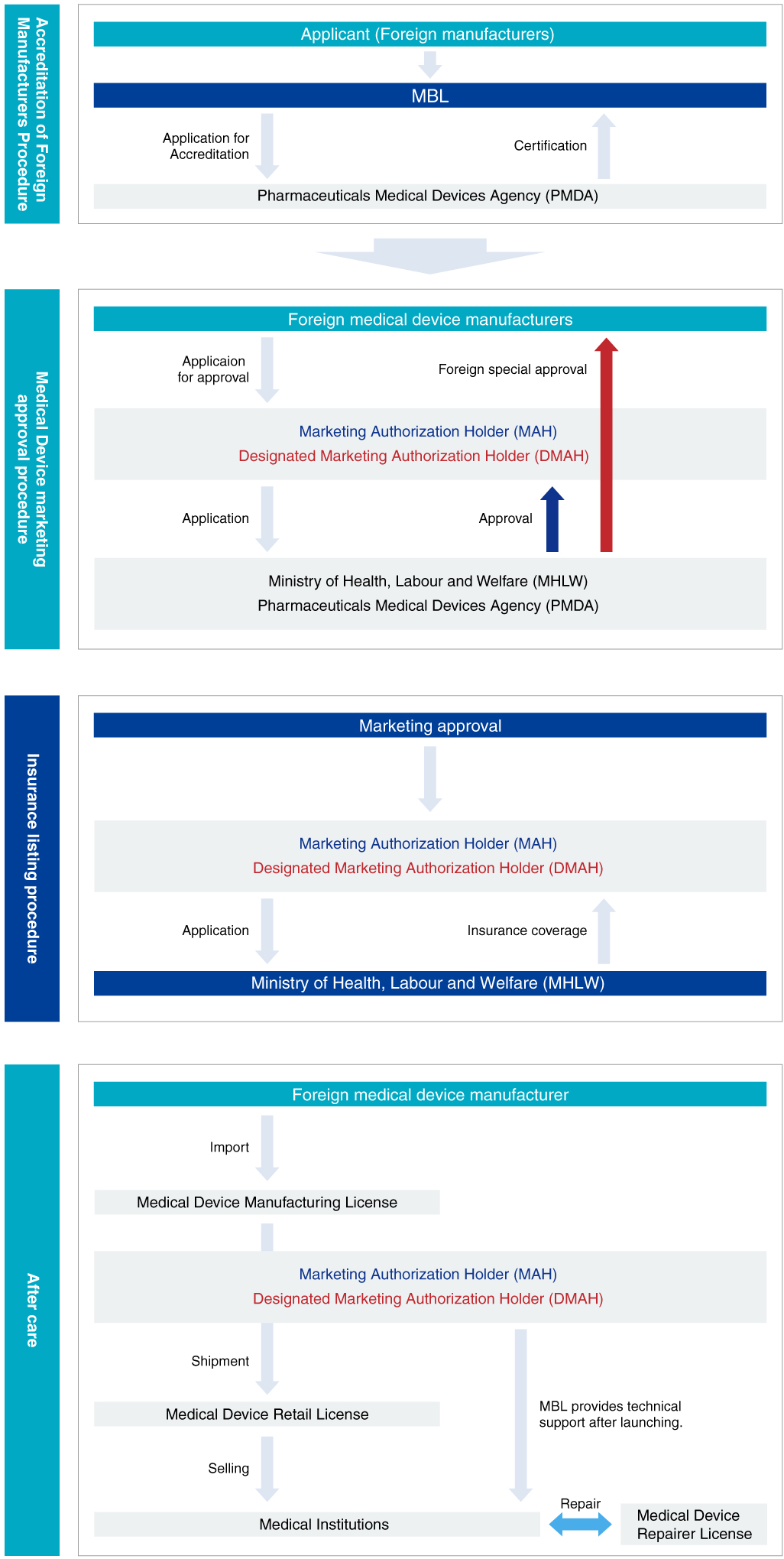
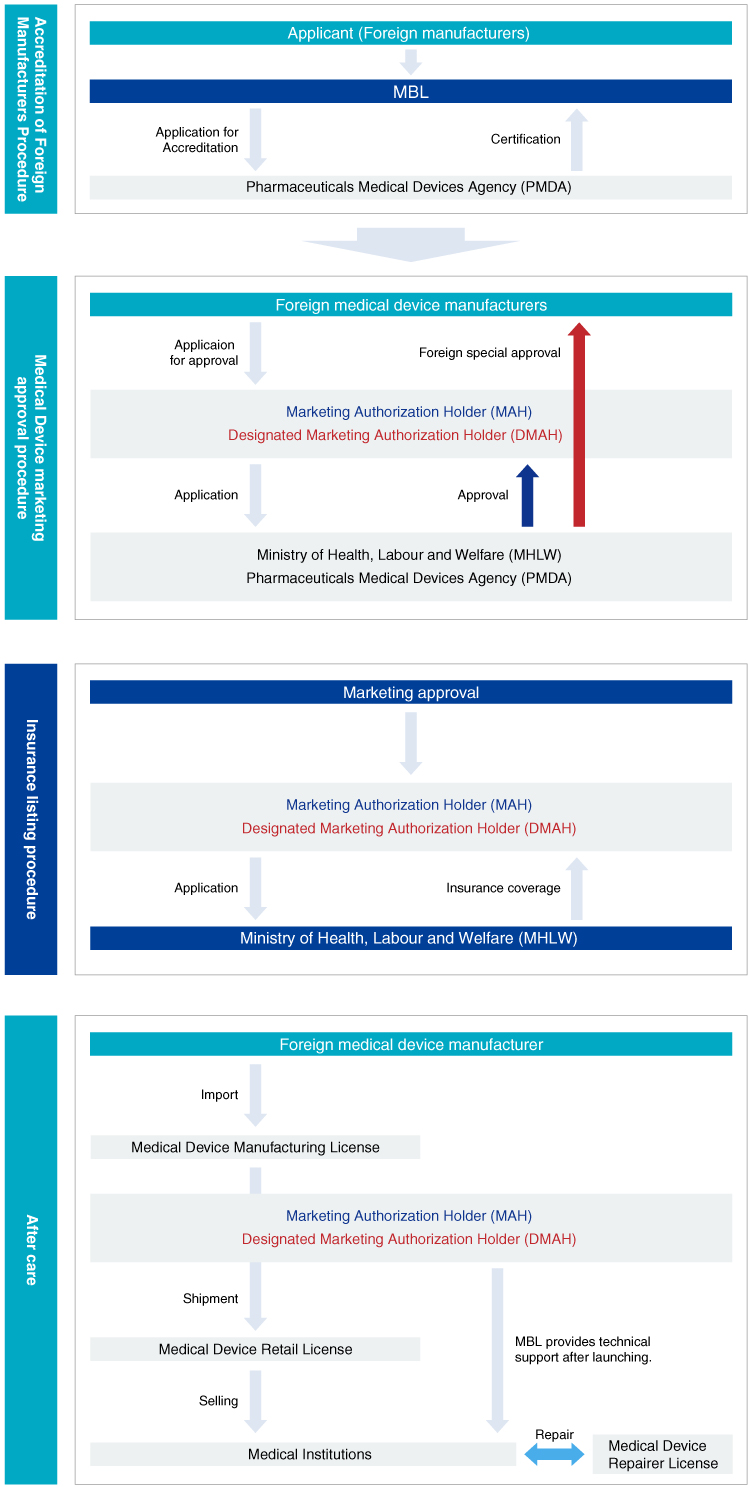
Japan does not have an LDT framework.
In the United States, there is a framework for IVDs for medical reimbursement coverage by conducting tests in hospitals and reference laboratories using medical devices and reagents that have not been approved as in vitro diagnostics. Such a test is called a laboratory developed test (LDT). On the other hand, in Japan, basically, health insurance is not covered unless tests are performed using approved medical devices or in vitro diagnostic products.
Medical devices and diagnostic products are required regulatory approval based on the Pharmaceuticals and Medical Devices Law (PMDL) to enter the Japanese market. This law specifies that in order to sell medical devices in Japan, foreign manufacturers must first be registered as an accredited foreign manufacturer. Next, they must make appoint with a Marketing Authorization Holder (MAH) or Designated Marketing Authorization Holder (DMAH) to apply their products to the Pharmaceutical and Medical Devices Act (PMD Act). These MAHs and DMAHs are obligated to perform quality control based on QMS and post-marketing safety management based on GVP, as well as various other operations such as product application.
MBL has all business licenses for medical device manufacturing, marketing, and sales of IVD/CDx products and repairing of medical devices. So we can strongly support foreign manufacturers to entry into Japanese market.
 |
Company Ⅰ | Company Ⅱ | Company Ⅲ | |
|---|---|---|---|---|
| Development | ||||
| Clinical performance trial | ||||
| PMDA approval | ||||
| Insurance listing | ||||
| Manufacturing | ||||
| Retailing | ||||
| Repairing |

One-stop support covers development through manufacturing, sales. You can leave flexibly whatever you want.
2. New Product Development
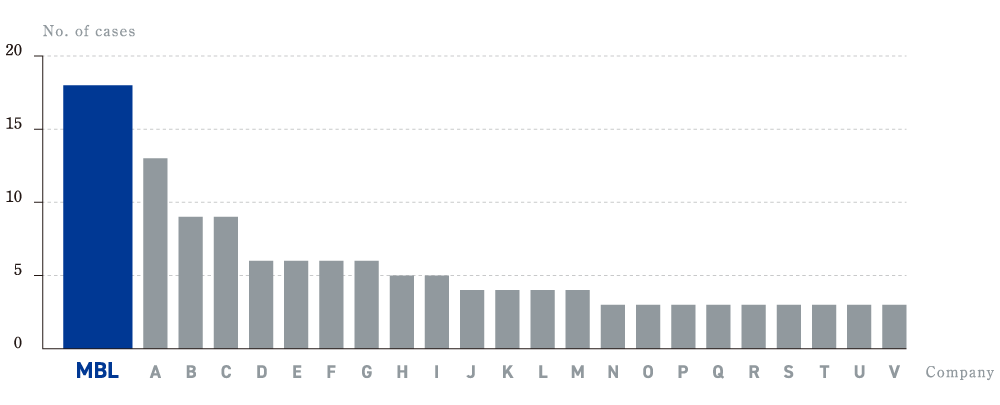
for the number of new IVDs medical reimbursement approval
Applications for diagnostics to be covered by the health insurance system fall into three categories: E1, E2, and E3. E1 is diagnostics with previously existing measurement items and measurement methods, E2 is diagnostics with new measurement methods, and E3 is diagnostics with new measurement items. At MBL, we work with all three categories, but we are particularly strong in E3.
Number of category E3 diagnostics covered
by the health insurance system from Feb 2003 to Feb 2023
Success Stories
Application and approval of CDx for Zolgensma in Japan
MEBCDX AAV9 TEST
Quick approval of Multiplex assay
MEBGEN RASKET-B Kit
A expedited review was recommended because of clinical necessity.
MEBRIGHT NUDT15 Kit
Development of FCM-based CDx in half years
CCR4 Protein Detection Kit (FCM)

Reliable with proven MBL
3. Strong relationship with KOLs
In Japan, in order to widely disseminate drugs and medical devices, applying for regulatory affairs is not sufficient enough. They also need to be recommended, by authoritative doctors in each field.
For example, MBL is the only IVD manufacturer participating in SCRUM-Japan, a consortium established by Japanese core hospitals and pharmaceutical companies to deliver optimal medical care to every cancer patient. MBL can anytime obtain advice from SCRUM-Japan, so that we does conduct appropriate clinical researches and regulatory affairs of IVD/CDx products. Also, through SCRUM-Japan, our medical devices can be launched on the market as recommended reagents by authorities.
"THE ONLY ONE IVD COMPANY" participating in SCRUM-Japan
SCRUM-Japan
The joint industry-academia Nationwide cancer genome screening project "SCRUM-Japan" is the world’s most advanced project of genetic alteration screening project for patients with cancers, undertaken by the National Cancer Center Hospital East in collaboration with medical institutions(>260) and pharmaceutical companies(12) all over the country, in the aim to provide the best medical care to every patient.
- Medical institutions: >260
- Pharmaceutical companies: 12
- Clinical data base: >10,000 patients
MBL can support your business with
"a wide range of KOL network".
Only one company
among IVD makers


Success Stories
Quick approval of Multiplex assay
MEBGEN RASKET-B Kit

"ISO 13485" Certification
MBL supports you with an extensive network of KOLs!
FAQ
MBL can meet a wide range of needs.
- We want to develop a CDx for our own medicines in Japan.
-
MBL can handle seamlessly from assay system development, to application, manufacture, and sales.
- Can you develop and launch CDx concurrently with our drug approval?
-
MBL provides consultation for simultaneous release.
- We want to measure a biomarker, but don't have the measurement system for it.
-
Based on a wealth of know-how in developing diagnostics, MBL will construct the best optimal measurement system.
- We want to sell diagnostics approved overseas in Japanese market.
-
Of course, only application work is available. Please leave it to MBL for manufacture and sales as well.
