Antigen and Antibody-Production Technologies
- Artificial Lymph Node Technology
- Human Monoclonal Antibody-Producing Technology
- Production of Human Monoclonal Antibodies by Phage Display Method
- SPYMEG, Fusion Partner Cell Line for fully Human Monoclonal Antibodies
- MAGrahd method
- Recombinant Protein Production Technology
- Antigen Production Technology
- Special Animal Monoclonal Antibodies
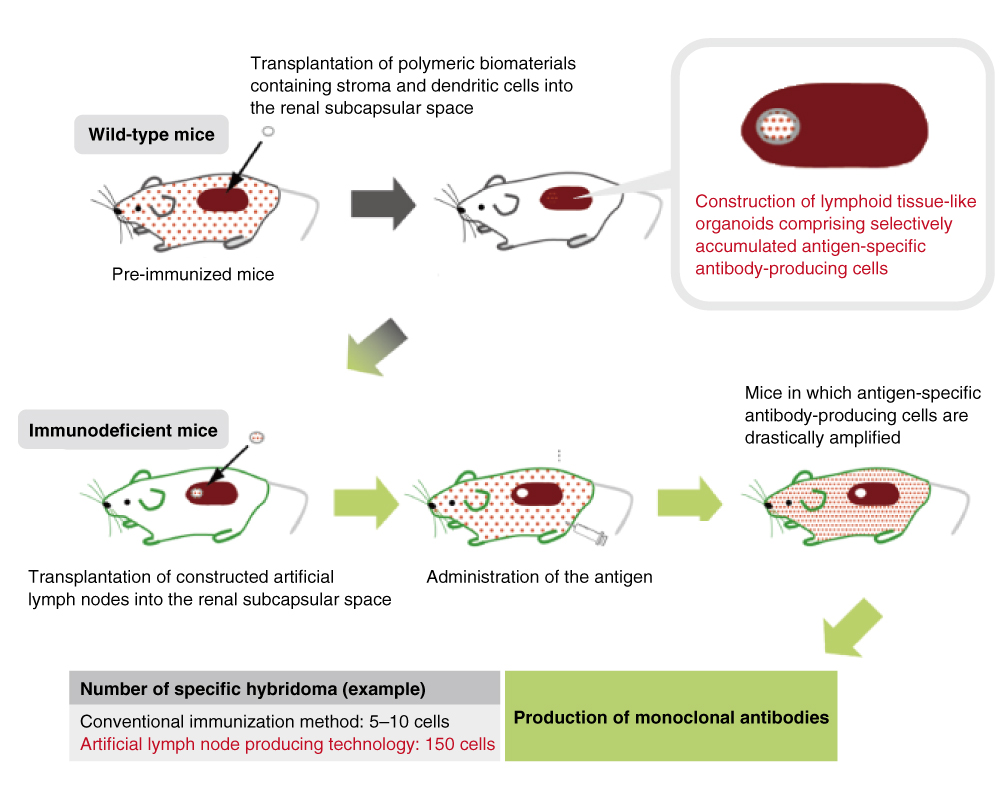
Artificial Lymph Node Technology
MBL’s artificial lymph node technology is a highly efficient antibody-production technology based on the method created by Professor Takeshi Watanabe at the National Research and Development Institute, RIKEN, and his associates. The technology was developed for the preparation of artificial lymph nodes in the kidney of mice. Normal lymph nodes contain immune cells that respond to various antigens, whereas artificial lymph nodes have a distinct feature in that they consist of only targeting antigen-specific immune cells. When artificial lymph nodes formed in immunized mice are transplanted into immunodeficient mice, they are capable of producing an antibody titer for target antigens that is 10 to 100 times higher than that in normal mice.1)
In monoclonal antibody-production using this technology, the number of monoclonal antibodies obtained is more than 10 times larger than what can be done by conventional methods. Also this MBL technology can provide antibodies with extremely high binding affinity.
1) Suematsu S. and Watanabe T. Nature Biotechnology. 22:1539-1545 (2004) (PubMed: 15568019)
Human Monoclonal Antibody-Producing Technology
Currently available antibody drugs include humanized antibodies and fully human antibodies generated from human genes. Since human antibodies are not recognized as foreign substances in the human body, they are considered to be highly effective and safe treatments. There are different methods for producing fully human antibodies: phage display technology, immunization of humanized transgenic mice which are genetically engineered to produce fully human antibodies, sequencing analysis of antibody genes from human antibody-producing cells, and a technology to fuse antibody-producing cells and fusion partners. MBL employs the phage display method and fusion partner technology (using the fusion partner cell line, SPYMEG) to efficiently obtain human monoclonal antibodies as the “seeds” of antibody drugs by making the best use of the advantages of each of these technologies.
Production of Human Monoclonal Antibodies by Phage Display Method*
The phage display method is a procedure to obtain antibodies that bind to target molecules (antigens) by presenting antibodies (e.g., Fab scFv) to fibrous phages of E. coli. With this technology, vast antibody libraries including a very broad variety of antibodies can be constructed, so extremely rare and high affinity antibodies can be obtained quickly and efficiently. The technology is also useful for producing antibodies against highly lethal toxins and viruses because it is possible to construct antibody libraries without the laboratory work of animal immunization with antigens. MBL owns a large number of human antibody libraries including 1 to 10 billion different antibodies, and we have succeeded in developing tumor-specific (suppressant) antibodies and virus neutralizing antibodies. In addition, utilizing the advantages of the phage display method in finding rare antibodies, we are developing methods for obtaining antibodies against G protein-coupled receptors (GPCRs) or glycosylated epitope - which, despite being useful drug discovery targets, are very difficult to produce antibodies for - and methods for efficiently selecting antibodies from vast antibody libraries by Next Generation Sequencing (NGS) and data science.
* A phage is a virus that infects specific bacteria. An antibody phage displays antibody fragments (i.e., Fab and scFv) that have antigen-binding ability on fibrous phages of E. coli. The phage contains the gene of the antibody displayed. By selectively obtaining the specific antibody phage against a target molecule (antigen) from a population of antibody phages that bind to various molecules, our technology can thereby also obtain the gene of the target antibody. In addition, it is possible to prepare antibody fragments (scFv and Fab), IgG proteins, etc. from acquired antibody genes.
SPYMEG, Fusion Partner Cell Line for fully Human Monoclonal Antibodies
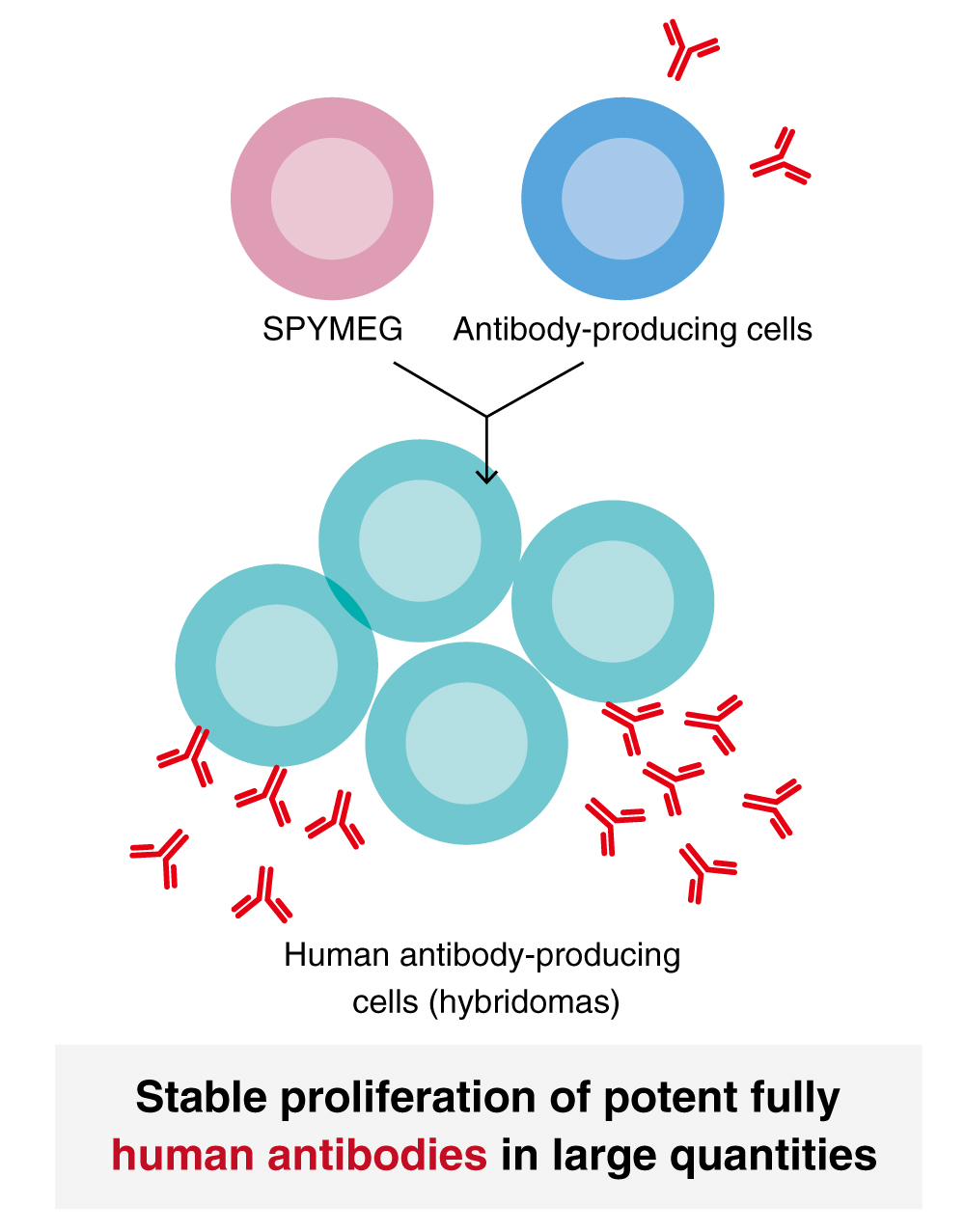
SPYMEG is a fusion partner cell line developed to obtain fully human monoclonal antibodies in collaboration with Professor Naomasa Yamamoto at the School of Pharmaceutical Sciences, Ohu University. The fusion of SPYMEG cells with antibody-producing cells collected from human blood facilitates extremely efficient and convenient acquisition of fully human antibody-producing hybridomas. Hybridomas developed from any animal cells except mice and rats often lose their antibody-producing ability over a course of long-term culture; however, hybridomas developed from SPYMEG cells and human antibody-producing cells can be stably cultured over a long period of time, and so there are a great many antibodies obtained in large quantities. SPYMEG is far superior in success rate to any of human antibody fusion partner cells reported to date. In addition, antibodies developed by using SPYMEG cells, which are fully human antibodies generated in the human body, can be expected to be applied to a very wide range of immunological research, not only for discovery of therapeutic antibodies, but also for analysis of antibody functions in human body, identification of diseases-specific antigens, searching for candidate vaccines, etc.
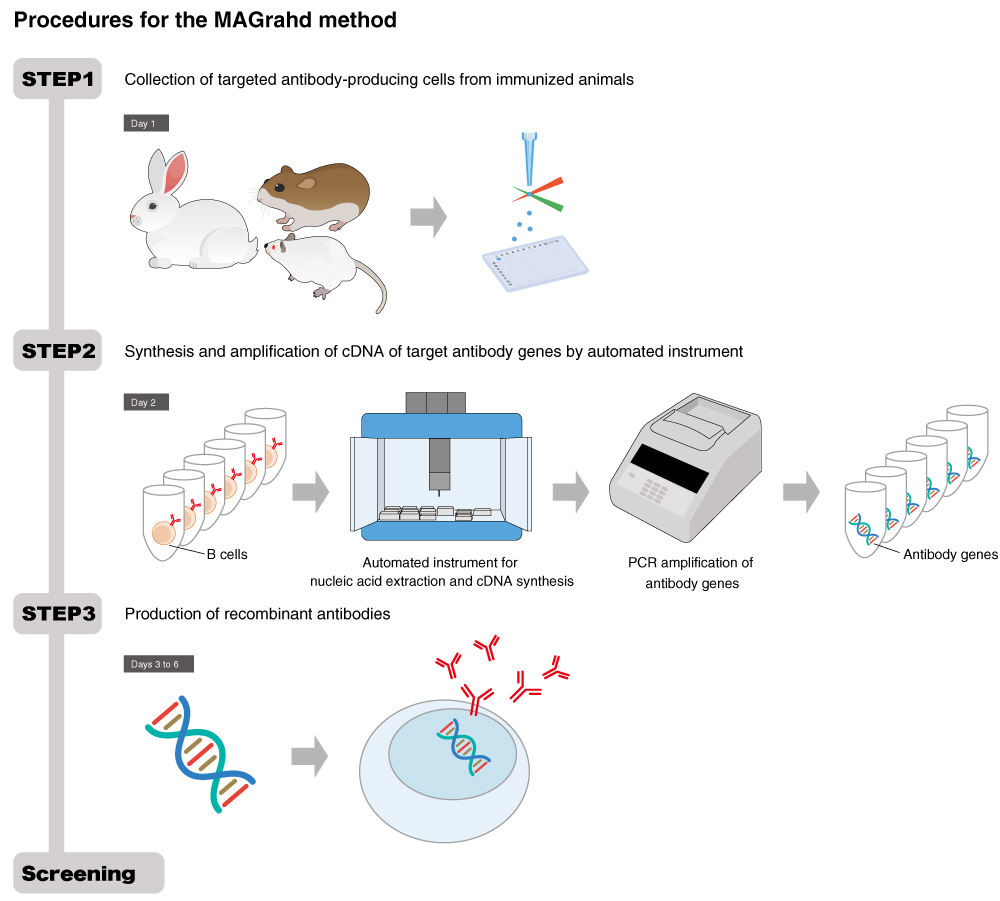
MAGrahd method
The MAGrahd method is developed for use in the analysis of antibody genes collected from one antibody-producing cell by Professors Masaharu Isobe and Nobuyuki Kurosawa at the University of Toyama. The method can isolate antibody-producing cells and express antibodies within just 5 days. It is one of the world’s fastest antibody acquisition methods. The MAGrahd method also ensures highly stable production of antibodies, since the method acquires antibody genes and then express recombinant monoclonal antibodies, which is the current mainstream method of producing antibody drugs. In addition, the method makes it possible to acquire rabbit monoclonal antibodies, which can provide very high affinity antibodies, and guinea pig monoclonal antibodies to help the development of high-quality antibodies that cannot be obtained with conventional mouse monoclonal antibodies.
<References>
・ Kurosawa N. et al. BMC Biol. 28;10:80. (2012) (PubMed: 23017270)
・ Kurosawa N. et al. Sci Rep. 29;6:25174. (2016) (PubMed: 27125496)
Recombinant Protein Production Technology
MBL is manufacturing recombinant proteins in-house for use as raw materials for diagnostic reagents. The manufacturing of those recombinant proteins requires the preparation of proteins that have the same three-dimensional structure as that of native human proteins in large quantities and at a high level of purity. We are investigating optimal procedures and conditions of production for the preparation of high-purity proteins, such as in-house development of purification columns and introduction of high-density cultivation systems for increased productivity, using animal cells, insect cells, and E.coli. In addition, we are working on further improvement of processing procedures for the improvement of production efficacy and protein storage stability by introducing new measuring equipment and analyzing the physical characteristics of proteins.
Antigen Production Technology
The quality of antigens is another critical issue in the development of high-quality antibodies. MBL is capable of obtaining antibodies that specifically react with native proteins by using high-quality recombinant proteins, with the same high level of purity as the raw materials used for diagnostic reagents, as antigens for developing antibodies. In addition, we are developing immunogenicity-enhancing technology to prepare optimum antigens necessary for antigens that lack immunogenicity. We also introduce the AI-based Epitope Prediction Algorithm (MODELAGON™) from MTI Ltd. for the production of antibodies that bind to specific sites (epitopes) of native human proteins.
→ Press release on the introduction of MODELAGON™ (Japanese)
Special Animal Monoclonal Antibodies
Antibodies are created against particular foreign substances that have entered the animal body, so antibodies are more readily produced against proteins derived from distinctly different animal species. For example, proteins that are highly homologous between humans and mice may be poorly homologous between humans and chickens, so chickens may be considered suitable for obtaining more potent antibodies. MBL has developed various methods using mice, rats, hamsters, rabbits, guinea pigs, chickens, and sheep to obtain monoclonal antibodies for highly homologous proteins. Moreover, antibodies in some animals are known to have different structures characterized by high chemical and thermal stability, such as camelid antibody (VHH) and shark antibody (IgNAR). We are capable of developing antibodies of various animal species by a use of phage display and genetic modification technologies. We are also exploring search on antibody structures that have novel biological functions.
