Strengths of MBL
MBL operates all processes required for manufacturing in vitro diagnostics products, from R&D to clinical performance study, to regulatory authorization application, to manufacturing and marketing. This system builds a close and trusted partnership with KOL physicians and achieves speedy commercialization.
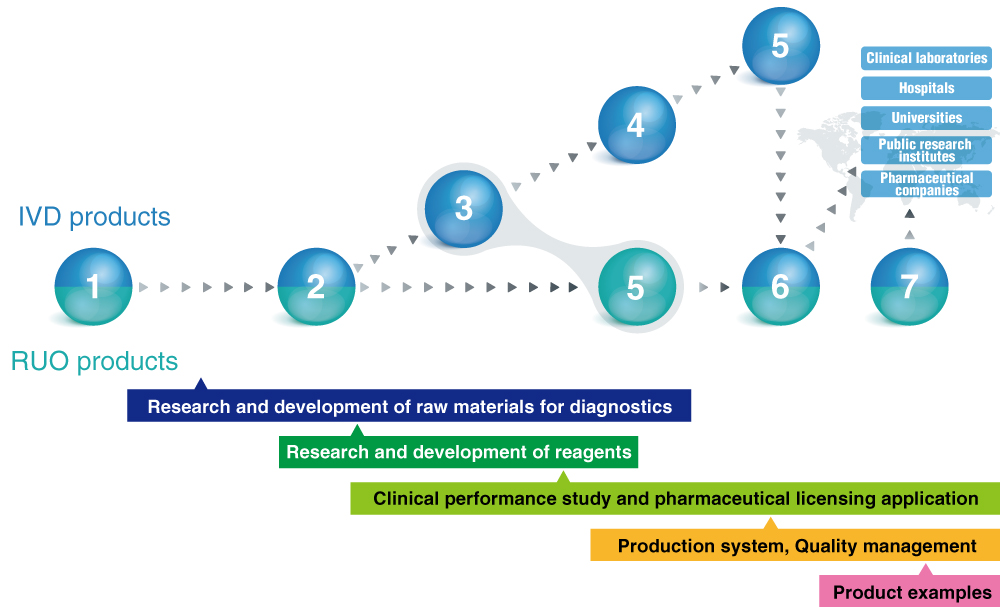
1: Market research and survey 2: Research and development 3: Clinical development
4: Regulatory approval 5: Commercialization of products and technologies
6: Manufacturing and sales 7: Technical Support
- Research and development of raw materials for diagnostics
- Research and development of reagents
- Clinical performance study and pharmaceutical licensing application
- Production system
- Quality management
- Product examples
