Product examples
In vitro Diagnostic reagents for the detection of RAS and BRAF gene mutations in colorectal cancer
MEBGEN RASKET™-B Kit
MEBGEN RASKET™-B Kit is designed for simultaneous detection of 48 kinds of RAS gene mutations*1 and BRAF (V600E)*2 gene mutations in clinical specimens from colorectal cancer patients. MBL had for some time manufactured and marketed MEBGEN™ RASKET Kit for the detection of RAS gene mutations. Since it has become increasingly important to confirm the presence or absence of BRAF (V600E) gene mutations for the treatment of colorectal cancer, the company developed a new version of MEBGEN™ RASKET Kit by incorporating BRAF (V600E) gene mutation detection assay into the kit.
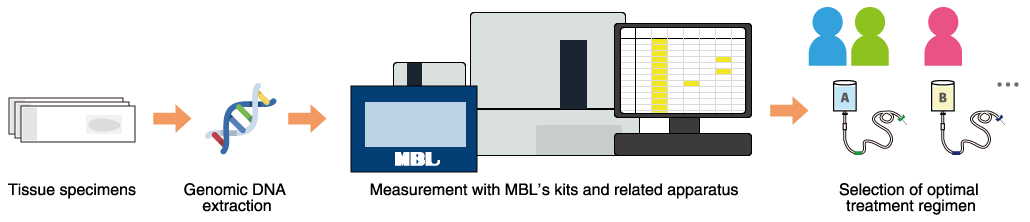
The kit is a valuable aid in determination of the presence of RAS or BRAF gene mutations prior to treatment and selection of the best treatment in colorectal cancer patients. Detection of mutations in BRAF (V600E) genes is also a useful aid in the treatment of Lynch syndrome*3 in colorectal cancer. The development and subsequent regulatory approval of the kit were achieved through steady following-up of specified regulatory processes, including the conducting of multi-center clinical performance trials with input from some key opinion leaders*4 and repeated consultations with the government agency.
*1: A mutation that causes a change in 48 amino acids in exons 2, 3, and 4 of the RAS (KRAS and NRAS) genes (total 6 exons)
*2: A gene mutation in which the 600th amino acid, valine, in the BRAF protein is replaced with glutamic acid
*3: Hereditary colorectal cancer
*4: Key Opinion Leader : An expert with broad influence in the medical industry
The world’s first in vitro diagnostic reagent for detecting NUDT15 polymorphisms for use in prediction of serious adverse reactions of thiopurine in the treatment of inflammatory bowel disease and other disorders
MEBRIGHT™ NUDT15 Kit
Thiopurine preparations are frequently prescribed for the treatment of inflammatory bowel disease, leukemia, rheumatologic disease, etc. Although this drug is inexpensive and expected to be effective, it can cause very rare but serious adverse reactions. MEBRIGHT™ NUDT15 Kit is designed for the detection of NUDT15 polymorphisms, which are believed to be a factor in these serious adverse reactions. Assay results can identify patients at high risk of thiopurine toxicity and thereby help with the selection of an optimal treatment regimen.
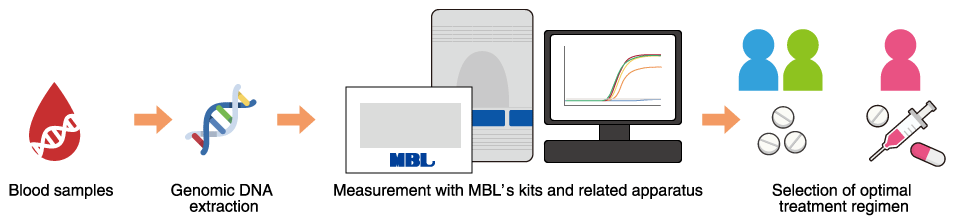
MEBRIGHT™ NUDT15 Kit was developed with the support of the Japan Agency for Medical Research and Development (AMED), which sponsored a research project, “Establishment of a framework for practical application of genomics for identifying thiopurine intolerant patients with inflammatory bowel diseases with a focus on the development of NUDT15 R139C genetic polymorphism test kit” (research representative: Kakuta Y, Tohoku University Hospital, coworkers: Abe Y et al.) in the Platform Program for Promotion of Genome Medicine. The kit is the first diagnostic reagent in the world for testing for NUDT15 polymorphisms.
It usually takes about 12 months after filing of an application to obtain marketing authorization for a diagnostic reagent. MEBRIGHT™ NUDT15 Kit was designated as a priority review item in a Review Meeting on Expedited Introduction of Medical Devices. etc. with High Medical Needs and received accelerated review and approval.
Strengths of MBL
- Research and development of raw materials for diagnostics
- Research and development of reagents
- Clinical performance study and pharmaceutical licensing application
- Production system
- Quality management
- Product examples
