Clinical performance study and pharmaceutical licensing application
MBL has the integrated corporate system to efficiently provide diagnostic reagents to the frontline of clinical practice by undertaking the complete product development process in a seamless manner, from planning of clinical performance study through regulatory application and subsequent listing in the National Health Insurance (NHI) Price List.
Timely, effortless delivery of diagnostic reagents to healthcare providers
New diagnostic reagents are required to pass through regulatory procedures before becoming available to medical professionals. These include the tests using specimens (i.e. clinical performance study*) to ensure that the reagents work as designed. Then, study (regulatory applications) are filed at the Ministry of Health, Labour and Welfare, with performance and safety data collected from clinical performance study, in order to obtain manufacturing/marketing licenses for in vitro diagnostic reagents. In addition, the marketing authorization holder is required to register products in the NHI Price List for reimbursement of costs to patients who wish to have testing with the reagents covered by their national insurance schemes.
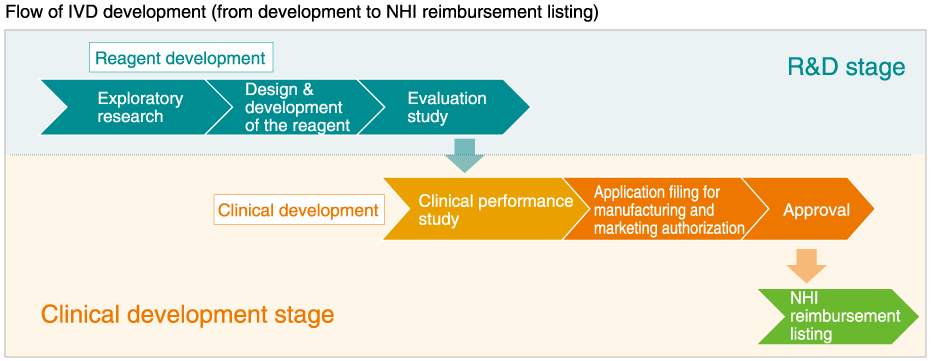
MBL boasts a track record of many successful regulatory applications for marketing approval and insurance coverage, and it continues to progressively launch new products year on year.
A characteristic of MBL’s business operations is its rapid and time-effective product commercialization system, covering everything from planning of basic research and clinical performance study through regulatory application and NHI price listing. This integrated system is supported by close and sustained communication with clinical experts, which is essential for the commercialization of innovative diagnostic reagents.
*Clinical performance study: study conducted using patient samples (blood, saliva, urine, stool) to verify the performance of reagent
Strengths of MBL
- Research and development of raw materials for diagnostics
- Research and development of reagents
- Clinical performance study and pharmaceutical licensing application
- Production system
- Quality management
- Product examples
