MESACUP™ anti-MDA5 TEST
Products may not be approved for sale as in vitro diagnostics in all countries.
Please contact us for further details.
Customers in the US, please contact MBL International Corporation for details.  ⇒
⇒
| Product | Code No. | Size | Method | RUO/IVD/ CE-IVD |
IFU | SDS |
|---|---|---|---|---|---|---|
| MESACUP™ anti-MDA5 TEST | 7874E | 96 wells | ELISA |
![]() : CE-marked In vitro Diagnostic Reagent
: CE-marked In vitro Diagnostic Reagent
Biomarker of dermatomyositis with rapidly progressive interstitial lung disease.
Clinically amyopathic dermatomyositis (CADM), dermatomyositis without muscle weakness, is associated with elevated rates of rapidly progressive interstitial lung disease (RP-ILD), which is extremely resistant to treatment. The prognosis is poor after respiratory status deteriorates. This shows the importance of detecting and diagnosing the disease at an early stage, when respiratory function is preserved, and initiating aggressive therapy.
Anti-MDA5 antibodies can facilitate a diagnosis of early dermatomyositis associated with RP-ILD. The ability to measure this antibody should improve the prognosis of patients with refractory pathologies.
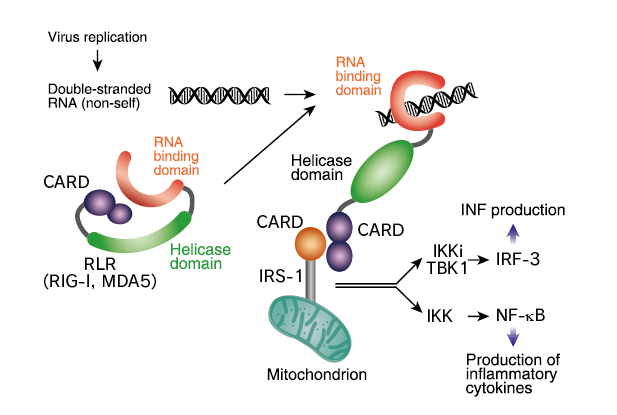
Melanoma Differentiation Associated Gene 5 (MDA5) protein
This protein belonging to the retinoic acid inducible gene I (RIG-I) family. It functions in innate immune defense against infection especially with RNA viruses such as picornaviruses by inducing type I interferon.
Anti-MDA5 antibody distribution by disease
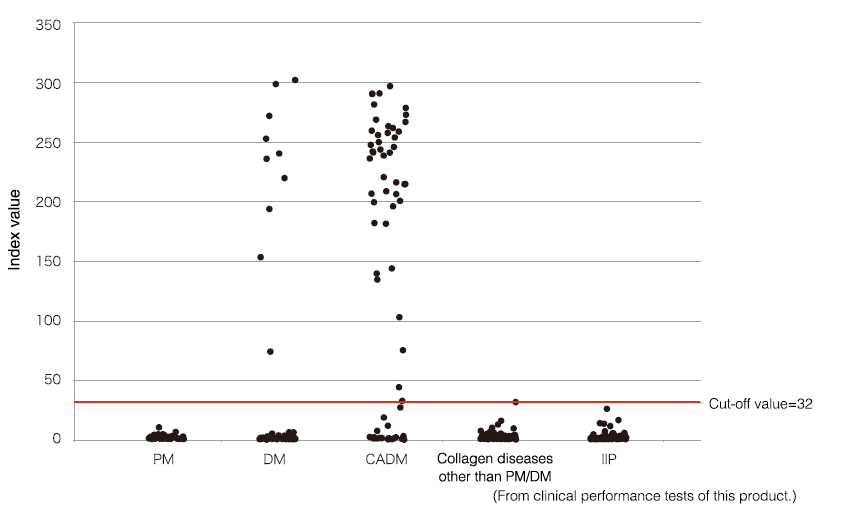
Anti-MDA5 antibodies are positive in 43 (65.2%) of 66 patients with CADM and in 10 (10.0%) of 100 with Classic DM (CDM). No samples tested positive for diseases other than DM, confirming that the reagent has very high disease specificity. Patients with positive anti-MDA5 antibodies have very high rates of complication with interstitial lung disease (50 (90.9%) of 55), and most of these patients developed acute (progression within 1 month) or subacute (progression in 1–3 months) of being diagnosed with the disease.
*Upper limit of measurement range is an index of 150. Values > 150 should be taken as references.
PM: polymyositis
IIP: idiopathic interstitial pneumonia
Measurement principle: Enzyme-linked immunosorbent assay (ELISA)
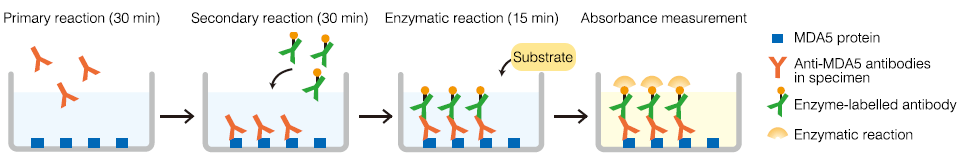
The MDA5 protein coated in microcups is reacted for 30 minutes with diluted patient samples. The cups are washed to remove unreacted antibody, then enzyme-labelled antibody is added and reacted for 30 minutes. The cups are washed again to remove unreacted enzyme-labelled antibody, then enzyme substrate is added and reacted for 15 minutes. The reaction is stopped, and the index value is calculated from the absorbance of simultaneously measured specimens and standards.
